Temperature and Density
| Reading: Ch. 2 sections 7 - 8 |
Homework: |
2.7, questions 72, 74, 76, 78*, 82* 2.8, questions 86, 90, 92*, 94*, 96, 100* |
* = ‘important’ homework question
Temperature
Background: There are three temperature scales in common use today . Can
you name them?

How were the end points of the two ‘metric’ scales
defined? In other words,
what natural conditions define these respective temperature values ?
 |
|
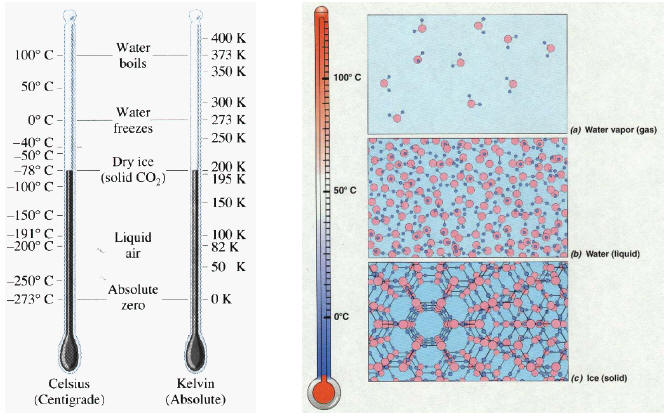
| The Centigrade and Kelvin Scales |
The Centigrade scale compared to the state of H2O |
Converting between Degrees Celsius and Kelvin
Task: By looking at the above graph , describe how the °C and K scales are
related. What do they have in common ? What is different ?
 |
|
 |
Simply add 273.15 to ANY temp. quoted in
°C to obtain the equivalent K value OR Simply subtract 273.15 from ANY temp. quoted in K to obtain the equivalent °C value |
Examples:
1.
What is 50°C in Kelvin?
2.
What is 200 K in Celsius?
Comparing the Fahrenheit , Kelvin and Celsius Temperature
Scales
Discussion: We saw that the end points for the °C scale corresponded to
specific
‘natural’ temperatures – the same is true for the °F scale. What
‘natural’
temperatures do you think 0 °F and 100 °F correspond to in
nature. How about 212
°F and 32 °F?

“You want to put
what, where?!..”
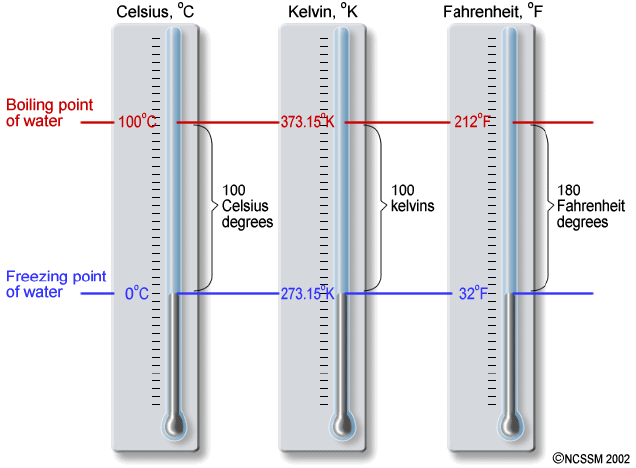
Diagram: Fahrenheit, Celsius and Kelvin thermometers side by side.
 |
Question: What is the obvious error in the above diagram? |
Task: By looking at the previous diagram , or the slide
provided, describe
how the °C and °F scales are related. What do they have in
common? What
is different ?
 |
The two basic differences between the
°C and °F
scales allow for equations relating them (conversion equations) to be constructed: |
For converting °C to °F:
For converting °F to °C:
Question: What is 90 °F in °C and in Kelvin?
Ask me about the extra credit temperature….
Temperature Ranges
 |
Discussion: If something is boiling, is it
necessarily ‘hot’? If it is frozen, is it necessarily ‘cold’? |
Task: View and make brief notes on the ‘temperature scale’
slide. Think of
the ‘hottest’ and ‘coldest’ things you come into contact with
on
a daily basis
– where do they fit into the ‘bigger picture’?
Density
| NOTE: THE FOLLOWING IS A REVIEW OF THE MATERIAL
YOU WILL LEARN DURING LAB #2. |
Review: How was the property of density defined during a previous lecture?
|
|
Density: |
Where: ‘amount of matter’ = _______________
Discussion: What is the S.I. unit of density? Is this a convenient unit?
=> Density =
__________________________________
Question: What are the two ‘convenient’ derived S.I. units of density used
by
chemists?
| Prev | Next |