SQUARE ROOT SIMPLIFY EQUATIONS CALCULATOR
factor calculator for a quadratic equation
,
Solving Non linear differential equations
,
difference between evaluation & simplification of an expression
,
algebra 2, vertex form of a linear equation
,
exponent definition quadratic hyperbola parabola
,
maths, algebra balancing linear equations
,
solving simultaneous nonlinear equations matlab
,
simplifying square roots with exponents
,
solve and graph non liner system of equations
,
solving second order non homogeneous differential equations
,
calculator texas instruments convert decimals into fractions
,
symbolic method math formula solving
,
a help sheet explaining how to solve equations by balancing them
,
finding the least common denominator algebra
,
quadratic equations vertex and standard form online calculators
,
easy addition and subtraction of algebraic expressions
,
solve non homogeneous first order partial differential equation
,
simultaneous equation solver quadratic 3 unknowns
,
Solving simultaneous algebra equations
,
convert decimal to radical fraction expression
,
solving linear equation cheats
,
factor polynomial college algebra two variable ax by
,
interactive games solve a quadratic equation by completing the square
,
ways to cheat on dividing a decimal by a whole number
,
solved sample papers for class viii of chapter square and square roots
,
square root calculator using simplified radical
,
simplifying radical expressions solver
,
factoring a difference of squares lesson plan for algebra 2
,
integer adding,subtracting,multiplying, dividing worksheet
,
Educational games solve a quadratic equation by completing the square
,
how to calculate greatest common divisor
,
writing linear equations powerpoint presentation
Thank you for visiting our site! You landed on this page because you entered a search term similar to this: square root simplify equations calculator. We have an extensive database of resources on square root simplify equations calculator. Below is one of them. If you need further help, please take a look at our software "Algebrator", a software program that can solve any algebra problem you enter!
|
|
|
|
|
| Initial Conc. (M) |
|
|
|
| Change in Conc. (M) |
|
|
|
| Equilibirum Conc. (M) |
|
|
|
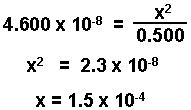
The change is only 0.03% of the initial value and is negligible.
[HA] = 0.500 - 1.5 x 10-4 = 0.500 M
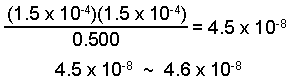
K and Q Lie on Opposite Sides of One (K>>Q or K<<Q)
When K is much larger than Q, or K is much smaller than Q, the change in amount of each species will be very large as the system moves towards a state of equilibirum. When this occurs, finding the change in concentration can often be facilitated by doing the following:
- When K>>Q and K > 1, assume 100% conversion into products, followed by the back reaction to establish equilibrium. When K<<Q and K < 1, assume 100% conversion into reactants, followed by the forward reaction to establish equilibrium.
- Make an ICE chart to determine change and equilibrium quantities starting with those resulting from the 100% conversion.
- Substitute quantities into the equilibrium expression.
- Assume the change is near zero such that "[A] - x" is equal to "[A]."
- Solve for the variable.
- Check to see if the change is less than 5% of the maximum amount, or within the limits set by your instructor. If not, use the method of approximations, a programmable calculator, or other method to solve.
- Solve for the equilibrium concentrations if asked to do so.
- Check your work.
H2(g) + I2(g) ![]() 2 HI(g) Kc = 794 @ 298 K
2 HI(g) Kc = 794 @ 298 K
- Initially the [HI] = 0, so K >>Q and K is > 1. The change in the concentration of each species will be large so we calculate the quantity of product formed assuming 100% conversion.
- Make an ICE chart starting with the concentrations after the 100% conversion.
100% conversion will result in the formation of 1.24 M HI (1 to 1 to 2 proporation) with neither reactant remaining.
|
|
|
|
|
| Initial Concentration (M) |
|
|
|
| Change in Concentration (M) |
|
|
|
| Equilibrium Concentration (M) |
|
|
|